
Fluorochromes are essentially coloured dyes which accept light energy at a given wavelength and re-emit it at a higher wavelength. These two processes are called excitation and emission. The process of emission follows extremely rapidly, commonly in the order of one thousand-millionth (10-9) of a second, and is known as fluorescence.
The two most common excitation sources in flow cytometry today are the argon-ion laser at 488nm, and helium-neon laser at 633nm, and any fluorochrome reagents used in flow cytometry must have excitation wavelengths broadly co-inciding with one of these lasers. More sophisticated flow cytometers may also use UV lasers. By employing a series of filters, and depending on the arrangement of lasers fitted, modern flow cytometers can detect the light emissions from several different fluorochrome types.
For diagnostic and research applications HMDS uses 3-colour (argon-ion equipped) and 4-colour (argon-ion and helium-neon equipped) flow cytometers. Three-colour investigations use antibodies coupled to fluorescein isothiocyanate (FITC), R-phycoerythrin (R-PE), and R-PE:cyanine-5 (PE:Cy5) fluorochromes, with a fourth fluorochrome conjugate - allophycocyanin (APC) - used in special 4-colour investigations such as minimal residual disease detection and rare-event analysis.

The orange-red coloured R-phycoerythrin and the turquoise-blue coloured allophycocyanin phycobiliprotein fluorochromes are extracted at the HMDS laboratory from plant material. R-PE is extracted from the marine red-algae Corallina officinalis collected from the sea-shore during exposure at spring tide. APC is extracted from the cyanobacteria Anabaena flos-aquae, grown in the laboratory in aquaculture flasks. Both fluorochromes are purified using an extensive sequence of ion-exchange and gel filtration chromatography.
The tandem-dye R-PE:Cy5 is produced by chemically coupling the cyanine dye 'Cy5:18' (® Amersham Pharmacia Biotech) to R-phycoerythrin. FITC is obtained commercially.
| Fluorescein isothiocyanate is a yellow-green coloured low molecular weight dye which couples to proteins via reaction with primary amine groups at high pH. FITC is excitable at 488nm, close to its absorption maximum at 494nm, and produces maximum fluorescence emission around 520nm. | 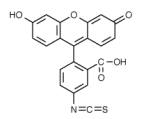 FITC |
Because of the large difference in molecular weight between FITC (389 Da) and immunoglobulin proteins (150,000 Da), simple gel filtration procedures are sufficient to separate free (unreacted) dye from FITC-labelled antibody.
More recently, a brighter and more photostable alternative to FITC has been developed by Molecular Probes, called Alexa®488, with similar excitation (494nm) and emission (519nm) maxima to FITC. We have found this dye to be up to six times brighter than FITC in some cases.
This arrangement of phycobiliprotein antenna pigments and chlorophyll is found in the phycobilisomes and allows light energy across much of the visible spectrum to be used for photosynthesis. This is particularly important in the marine environment where the maximum transmittance of sea-water is around 500nm, outside the optimum wavelengths for chlorophyll.
Phycobiliproteins are relatively large, and contain multiple chromophore prosthetic groups, which are responsible for the fluorescent properties of these proteins, and generally result in fluorochromes of very high efficiency, commonly in excess of 90% absorbed light energy transferred to chlorophyll.
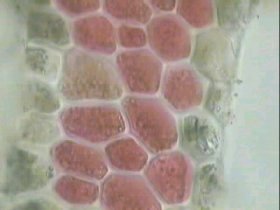 |
R-phycoerythrin is extracted at the HMDS laboratory from Corallina officinalis red algae, shown here at x100 magnification. Intact phycoerythrin-containing cells can clearly be seen, as well as some which have lysed during the extraction procedure, spilling their contents. During purification of R-PE, the only major contaminating protein is R-phycocyanin, which has dissimilar chromatographic properties to R-PE. |
 |
Allophycocyanin is extracted at the HMDS laboratory from Anabaena flos-aquae cyanobacteria, shown here at x600 magnification. The major contaminating proteins are chlorophyll and C-phycocyanin - C symbolising its cyanobacterial origin - both present in much greater amount than APC. C-phycocyanin has similar chromatographic properties to APC, significantly complicating the purification procedure. |
Optimum labelling efficiency for use in flow cytometry is between 5 and 8 Cy5 molecules per phycoerythrin molecule. Below a ratio of about 4:1 there is insufficient Cy5 to absorb all photon emissions from phycoerythrin, so light energy at 578nm begins to escape from the complex. This emission leakage can compromise the performance of a PE:Cy5-labelled reagent if used in combination with a phycoerythrin-labelled reagent.
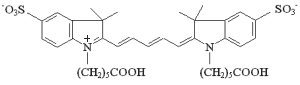 |
| Cyanine-5.18 molecular structure |
The four main chromophore types present in algal and cyanobacterial species are phycocyanobilin (PCB), phycoerythrobilin (PEB), phycourobilin (PUB), and cryptoviolin. R-phycoerythrin has both PEB and PUB chromophores, whereas APC has only PCB. Generally, larger chromophore aromatic ring structures are associated with longer emission wavelengths.
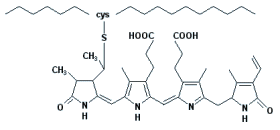 Phycoerythrobilin chromophore molecular structure |
PUB is prevalent in marine photosynthetic organisms, and absorbs strongly at 498nm, close to the maximum transmittance of sea-water at 500nm. PEB absorbs strongly around 540nm and is responsible for the 'shoulder' to the main 565nm absorbance peak of R-PE. The 565nm peak itself is a composite of both PUB and PEB chromophores. PCB absorbs mainly above 600nm, and produces the characteristic absorption maxima at 620nm in C-phycocyanin and 650nm in APC. The different absorption peaks seen in a typical phycobiliprotein wavelength scan reflects the interaction of different chromophores.
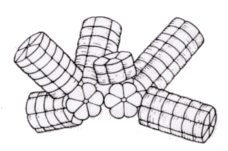
Morphology varies with species, but invariably consists of rods of stacked disks radiating from a central core. APC is always present in cyanobacterial and red-algal phycobilisomes together with either C- or R-phycocyanin, and usually one of the green-absorbing biliproteins C-phycoerythrin, R-phycoerythrin, B-phycoerythrin or phycoerythrocyanin.
APC and allophycocyanin-B constitute the core of the phycobilisome, with other biliproteins constituting the rods. Light energy is transmitted down the rods to the core, then to chlorophyll which is embedded in the 'thylakoid' membranes of the photosynthetic chloroplasts. The normal sequence of energy transfer is:
phycoerythrin - phycocyanin - allophycocyanin - allophycocyanin B - chlorophyll a
 |
 |
Rod-and-core structure of cyanobacterial phycobilisome. Left-hand diagram shows stacks of hexameric phycocyanin complexes comprising the rods. The right-hand diagram shows phycoerythrin- and phycocyanin-containing rods, with a three-cylinder core consisting of APC and APC-B. [Adapted from AN Glazer. Phycobilisome: a macromolecular complex optimized for light energy transfer. Biochemica et Biophysica Acta, 1984, p29-51] | |
Comments & feedback to: admin@hmds.org.uk