[ Home Page ] [ Site Map ]
The Myelodysplastic Syndromes
Definition
Clinical Features
Laboratory Diagnosis
Classification
Treatment and Prognosis
The myelodysplastic syndromes are acquired clonal disorders of myeloid pluripotent stem cells characterised by morphological abnormalities in the maturing cells of the granulocyte, erythroid and megakaryocyte lineages. Haemopoiesis is ineffective, giving rise to cytopenias. By convention they are separated from the acute myeloid leukaemias on the basis of the percentage of blasts present in the blood and bone marrow.
- Median age at diagnosis is ~70 years. 70% of patients are aged > 50 years.
- The exception is the monosomy 7 syndrome of children.
- Males affected more frequently than females.
- Characterised by cytopenias in the peripheral blood (except CMML)
- Symptoms reflect the cytopenias, i.e. anaemia, infection, bruising and haemorrhage.

Peripheral Blood
- Red cells: May show macrocytosis, anisopoikilocytosis and anisochromasia and basophilic stippling, especially in Sideroblastic Anaemia. Nucleated RBC may be present.
- Neutrophils: May show hypogranular cytoplasm, and abnormal nuclear segmentation; bilobed (spectacle) pseudo-Pelger-Huet nuclei, or round mononuclear nuclei, recognisable as mature by the coarse clumping of the chromatin. Gross hypersegmentation is occasionally present.
- Monocytes: In CMML they are increased (>1.0 x 109/l), may be larger than normal with poorly developed nuclei.
- Platelets: Platelet anisocytosis, giant platelets and nucleated megakaryocyte fragments may be present. Platelet count usually raised specifically in the 5q- syndrome.
- Blasts: Circulating blasts may be present.
Bone Marrow Aspirate
- Usually hypercellular. < 5% normo or hypocellular.
- Megakaryocytes: may be normal, increased or decreased. Abnormal forms:- hyperlobated mature forms, smaller forms showing nuclear lobe separation, very small (micro)megakaryocytes with mature chromatin and scanty platelet bearing cytoplasm.
- Granulocytes: granulocytes and precursors from the myelocyte stage onwards are usually hypogranular. Nuclear abnormalities as in the peripheral blood.
- Erythroid precursors: Bi- or multi-nucleate forms may occur at all stages of maturation. Nuclei may show abnormal lobulation or rosetting. Mitotic figures may be broken up. Cytoplasm in normoblasts may be ruffled, clefted or vacuolated. Basophilic stippling in normoblasts may be present.
- Blast cells: usually small, with scanty cytoplasm and few or no granules may be increased. Auer rods may be present.
- Eosinophils and basophils: rarely increased and may show non-specific abnormalities of the granules and nuclei.
Trephine Biopsy
- Usually hypercellular (better than the aspirate for estimating cellularity and megakaryocyte numbers)
- Architecture usually abnormal, especially disruption of the erythroid islands, clustering of the megakaryocytes. Blast cell clusters may be present (ALIPs).
- Dyserythropoiesis and abnormal megakaryocytes can be identified, but granulocyte dysplasia is better seen on the aspirate or in the blood.

Myeloperoxidase
(Sudan Black B gives identical results)
- Neutrophils may be negative.
- Auer rods seen twice as often as on the Romanowsky stain
- Blasts usually negative, occasionally positive
- Intermediate precursors usually stain normally
Combined Esterase
(chloroacetate plus a-naphthyl acetate esterases)
- Granulocytes may show abnormal double staining with both substrates.
- Micromegakaryocytes identifiable by ANAE positivity
- Monocytes identified by strong ANAE positivity
Perl's stain for iron
- Usually shows increased storage iron, reflecting ineffective erythropoiesis.
- Identifies ringed sideroblasts.
- Blasts are usually CD34+.
- Megakaryoblasts are CD42b and/or CD61 positive.
- Cells with abnormal phenotypes. Eg. CD34+/CD14+ may be present.
- Erythroid progenitors are Glycophorin positive.
- Blast cells may co-express antigens from more than one lineage.
- Clonal abnormalities are identified in 40-60% of cases by metaphase cytogenetics, confirming the morphological diagnosis of MDS.
- Monosomy 5 and 7, 7q- and 5q- are the commonest abnormalities found.
- Complex karyotypes (3 or more structural or numerical abnormalities) may be present, and are characteristic of MDS
- Fluorescent in-situ hybridisation (FISH) can identify marker chromosomes, translocations, trisomies, etc.

The current system for classifying MDS is that devised by the FAB group, but will probably be superseded shortly by new proposals currently under consideration. The FAB classification is based on morphology, peripheral blood monocyte count, the percentage of blasts in the blood and marrow, and the presence or absence of Auer rods.
FAB CLASSIFICATION OF THE MYELODYSPLASTIC SYNDROMES
| Morphological Subtype |
Peripheral Blood |
Bone Marrow |
| Refractory Anaemia (RA)* (12-43% of cases) |
Blasts <1% |
Blasts <5% |
| Refractory Anaemia with Ring Sideroblasts (RARS) (14-37% of cases) |
Blasts <1% |
Blasts <5%; >15% of NRBC are ring sideroblasts |
| Refractory Anaemia with Excess Blasts (RAEB) (13-43% of cases) |
Blasts <5% |
Blasts 5-19% |
| Refractory Anaemia with Excess Blasts in Transformation (RAEB-T) (4-27% of cases) |
Blasts >5% OR Auer rods present** |
Blasts 20-29% OR Auer rods present** |
| Chronic Myelomonocytic Leukaemia (CMML) (1-22% of cases) |
As any of the above but with >1 x 109/l monocytes |
As any of the above with or without an increase in monocytes and/or promonocytes |
* In about 5% of cases with neutropenia and/or thrombocytopenia, anaemia is absent, and the term 'Refractory Cytopenia' can be used.
** Auer rods, whether found on the Romanowsky or myeloperoxidase stain place the diagnosis in the RAEB-T category, irrespective of the blast percentages.
The classification and diagnostic criteria of the haematological malignancies, including the Myelodysplastic Syndromes, is currently under consideration and future developments may include the following:
- Discarding the category RAEB-T and classifying all cases with > 20% blasts as Acute Myeloid Leukaemia
- Transferring CMML to a new class of disorders, the Myelodysplastic/Myeloproliferative Diseases, which will also include Juvenile Myelomonocytic Leukaemia (JMML) and Atypical Chronic Myelogenous Leukaemia, BCR/ABL- (aCML).
- Confining the term Refractory Anaemia (RA) to cases where only the red cell progenitors appear dysplastic.
- Retaining the category Refractory Anaemia with Ringed Sideroblasts (RARS).
- Creating a new category Refractory Cytopenia with Multilineage Dysplasia (RCMD).
- Retaining the category Refractory Anaemia with Excess Blasts (RAEB).
- Creating the category 5q- syndrome.
- Creating the category Myelodysplastic Syndrome, unclassifiable.
 |
 |
 |
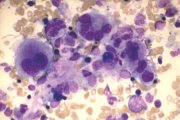
Dysplastic megakaryocytes. Variable size, nuclear lobe separation. |
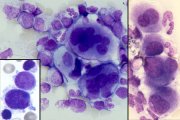
Dysplastic megakaryocytes. Hyperlobated nuclei (centre). Micromegakaryocytes with dense nuclear chromatin (inset left). Immature megakaryocytes with nuclear lobe separation (right). |
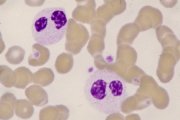
Circulating pseudo-Pelger neutrophils. Agranular cytoplasm, bilobed 'spectacle' nuclei. |
 |
 |
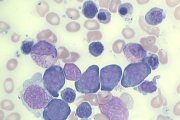 |
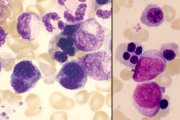
RAEB. Note agranular myelocytes and agranular poorly segmented neutrophil, and abnormal basophil with dense nuclear chromatin (arrowed). |
Therapy-related MDS (left). Gross dyserythropoiesis. RAEB-T (right). Two myeloblasts, one with an Auer rod, and a quadrinucleate normoblast. |
RARS. Left-shifted megaloblastoid erythropoiesis. |
|
|
RARS. Perl's stain showing gross sideroblastic change. |
CMML. Peripheral blood showing 5 over-large mature monocytes with poorly formed nuclei and 2 agranular poorly segmented neutrophils. |

TREATMENT
- Supportive, with red cell transfusion, platelet transfusion and antibiotics as required.
- Erythropoietin may increase the haemoglobin if the endogenous levels are < 100u/ml.
- GCSF may increase the neutrophil count, but does not affect long term prognosis.
- Allogeneic BMT is curative in 20-30% of eligible patients.
- Intensive chemotherapy may induce remissions in RAEB-T.
PROGNOSIS (MEDIAN SURVIVAL)
| RA | 50 months |
| RARS | 51 months |
| RAEB | 11 months |
| RAEB-T | 5 months |
| CMML | 11 months |
TRANSFORMATION TO ACUTE LEUKAEMIA
| RA | 12% |
| RARS | 8% |
| RAEB | 44% |
| RAEB-T | 60% |
| CMML | 14% |
It is likely, that given enough time, all forms of MDS would progress to acute leukaemia. Death from the complications of marrow failure, or intercurrent illness in the age group affected, determine the percentage of patients who develop overt leukaemia.

[ Home Page ] [ Site Map ]
Copyright © HMDS 1999-2005
Document last updated:
Tuesday, 18 November 2003
Comments & feedback to:
admin@hmds.org.uk
[URL: www.hmds.org.uk/mds.html]